WE OFFER ADVANCED ORTHOBIOLOGICS, including ADULT MESENCHYMAL STEM CELL THERAPY, PLATELET-RICH PLASMA (PRP), Nerve Hydrodissection, prolotherapy, and OTHER SERVICES.
We provide advanced autologous orthobiologic and regenerative medicine services, including patient-derived mesenchymal stromal cell–containing biologic therapies, platelet-rich plasma (PRP), nerve hydrodissection, prolotherapy, and related interventional treatments.
Our clinical approach is grounded in integrative and regenerative medicine principles that leverage the body’s intrinsic repair mechanisms, using minimally manipulated, autologous biologic products where appropriate and in compliance with current regulatory frameworks. These therapies are intended to support tissue repair, modulate inflammation, and promote functional recovery through biologically mediated processes rather than through exogenous cell replacement.
Orthobiologics represent a rapidly evolving area of musculoskeletal and regenerative care, supported by an expanding body of translational and clinical evidence. Advances in cell characterization, biologic processing, and delivery methodologies continue to broaden the potential applications of autologous biologic therapies while emphasizing safety, traceability, and regulatory compliance.
Dr. Glowney is committed to delivering patient-centered, evidence-informed, and FDA-guidance-aligned autologous biologic care. Clinical decision-making prioritizes safety, functional restoration, pain reduction, and objective outcomes, with careful attention to appropriate patient selection and informed consent.
By integrating extensive clinical experience with contemporary research, we offer regenerative and integrative treatment pathways for orthopedic injuries, traumatic brain injury, post-viral syndromes, including long COVID, and other conditions where autologous biologic therapies may provide meaningful benefit.
Additional information regarding our clinical philosophy, regulatory approach, and available services can be found in the “About Us” and “Our Services” sections of this site.
To inquire about consultations or scheduling, please get in touch with our office at 1-720-550-6175.
orthobiologics at work
Before After
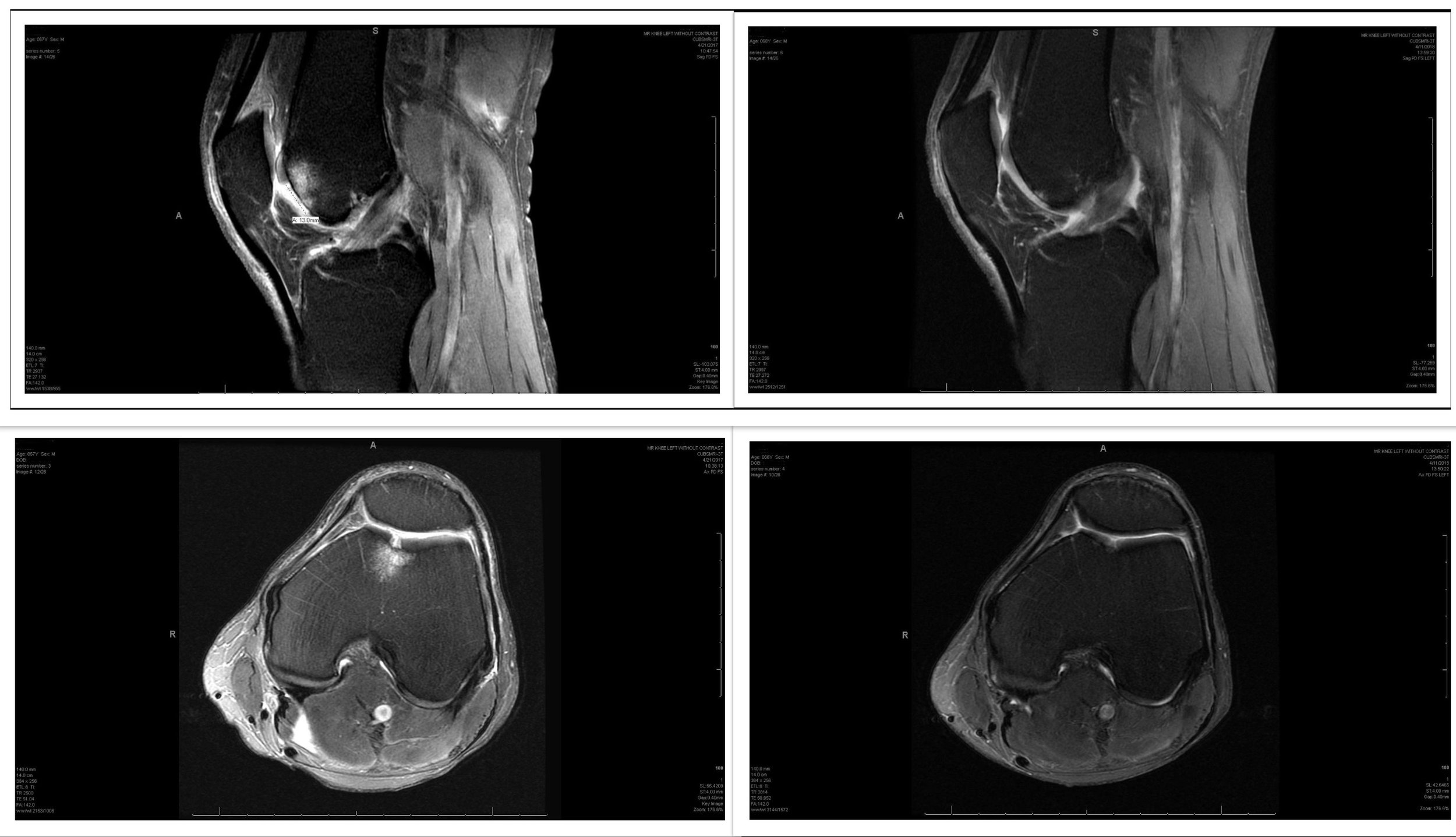
This is an example of a patient’s results from platelet-rich plasma (PRP) therapy at Boulder Biologics. Notice the cartilage regrowth and bone marrow edema improvement. We caution that these results are the exception and would not be expected in more advanced/diffuse arthritis patients but it highlights that PRP can positively affect cartilage injury in some patients.
Subchondral Autologous Biologic Therapy
In the accompanying image, Dr. Glowney is utilizing real-time ultrasound and fluoroscopic guidance to deliver an autologous, patient-derived cell-containing biologic product to both the intra-articular space and the subchondral bone of an arthritic joint. Image-guided delivery is employed to optimize anatomic accuracy, procedural safety, and reproducibility.
This therapeutic approach is designed for patients with focal and/or generalized osteoarthritis, in which pathology involves not only the articular cartilage and synovium but also the underlying subchondral bone. Contemporary osteoarthritis research increasingly recognizes the subchondral compartment as a critical driver of pain, inflammation, and structural degeneration.
Targeted delivery of autologous biologic material to both the joint space and subchondral bone is supported by clinical literature. Work by Hernigou and colleagues demonstrated improved clinical outcomes in osteoarthritis patients treated with autologous bone marrow–derived cell concentrates delivered to both compartments, compared with patients treated with intra-articular delivery alone. These findings underscore the importance of addressing the osteochondral unit as an integrated biological system rather than treating the joint space in isolation.
All procedures are performed using autologous biologic products and are structured to align with current FDA guidance regarding appropriate terminology, processing, and clinical use. No claims are made regarding exogenous cell replacement or unapproved biologic manipulation; rather, these interventions are intended to support endogenous repair mechanisms through targeted, image-guided delivery of patient-derived biologic material.
To learn more about our evidence-informed clinical protocols and treatment philosophy, please review the sections under “Our Services.”
Autologous Cell-Containing Biologic Processing and Characterization
Boulder Biologics employs controlled, protocol-driven procedures designed to obtain high-quality, viable, autologous cell-containing biologic material from a patient’s own bone marrow aspirate. These processes emphasize sterility, cell viability, traceability, and alignment with current regulatory guidance regarding appropriate terminology and clinical use.
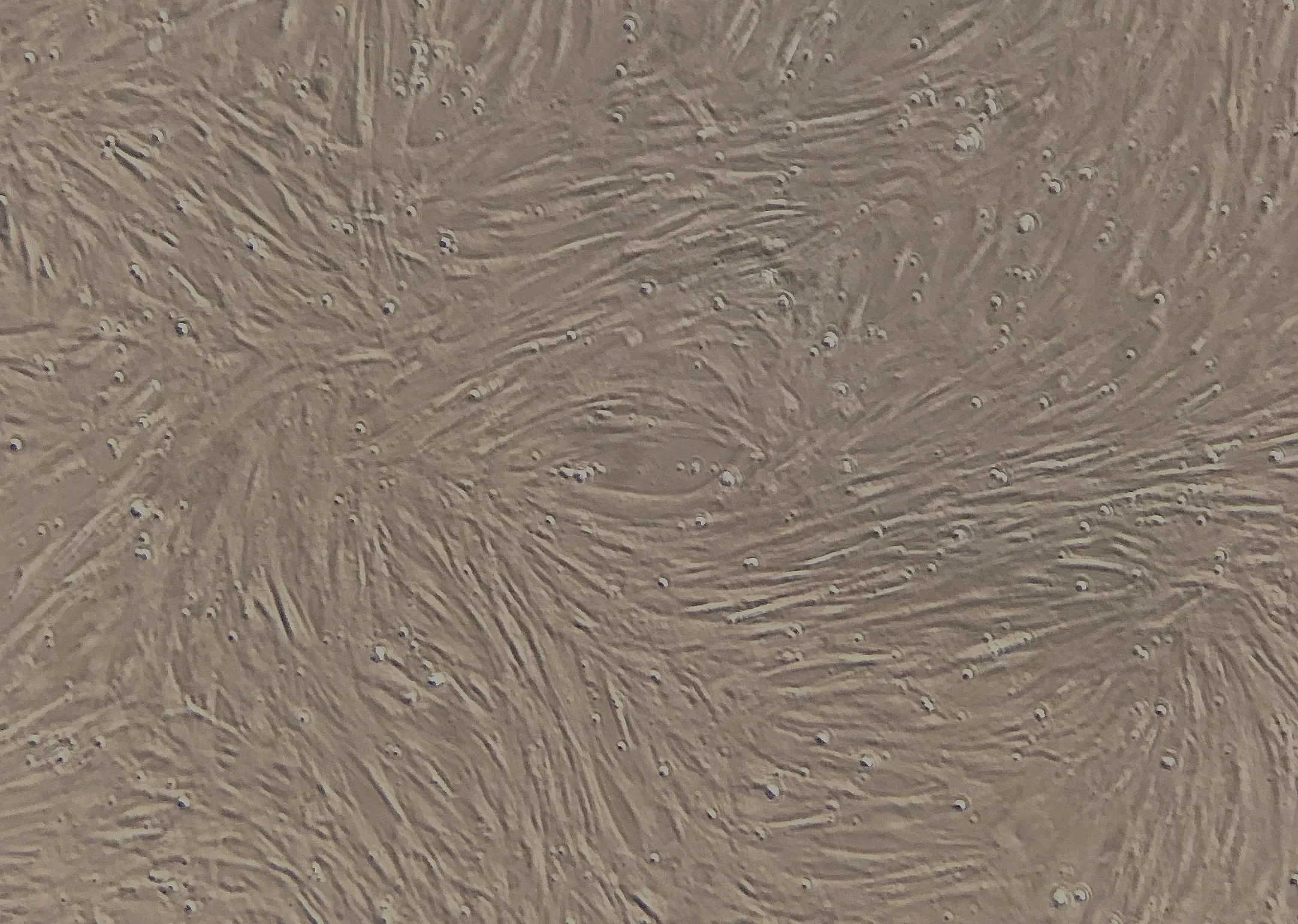
The image shows adherent, fibroblast-like stromal cells derived from an autologous bone marrow aspirate, visualized by phase-contrast microscopy during short-term culture. These cells represent mesenchymal stromal cell–enriched populations commonly observed following bone marrow–based processing and adherence selection. The cells shown are viable and metabolically active at the time of imaging.
For characterization and quality assessment purposes, cells are maintained under standard laboratory conditions using Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and incubated in a humidified atmosphere containing 5% carbon dioxide. Culture duration in this context is limited and intended solely for observational, research, and quality evaluation purposes, not for claims of lineage specification or therapeutic differentiation.
Consistent with FDA guidance, we avoid unsupported claims regarding “stem cell” replacement or regeneration. All biologic materials are autologous and patient-derived, and their clinical use is intended to support endogenous repair processes through biologically mediated signaling and microenvironmental modulation rather than through direct tissue engineering or exogenous cell engraftment.
This commitment to scientific rigor, appropriate terminology, and regulatory awareness underpins our broader approach to autologous orthobiologic therapies and translational regenerative medicine.